HEAT AND FIRST LAW
4B10.20 MIXING HOT & COLD WATER4B20.10 CONVECTION TUBE
4B20.20 TWO CHIMNEY CONVECTION BOX
4B20.25 CANDLE IN TUBE (Symmetry Breaking and Convection)
4B30.21 HEAT TRANSFER BY CONDUCTION IN METAL
4B30.22 HEAT TRANSFER BY CONDUCTION IN GLASS
4B30.23 CONDUCTION IN METAL VS FOAM
4B30.50 FROST FORMATION ON LIQUID NITROGEN CANNON
4B40.10 LIGHT MATCH THE HARD WAY (RADIATION) NOT CURRENTLY AVAILABLE
4B40.15 HEAT TRANSFER BY RADIATION
4B40.40 GOOD ABSORBERS & GOOD EMITTERS
4B50.20 HIGH HEAT CAPACITY OF WATER
4B60.10 KE TO HEAT USING LEAD SHOT
4B60.15 KE TO HEAT USING COLLIDING BALLS
|
4B10.20 Mixing Hot & Cold Water Use as example of what happens when you bring objects at different temperatures in contact and as example of irreversible process. Slowly add hot water until full. The top part will turn green where the water mixes.
Setup Requirements:Assembled as needed. You will need to allow time for hotplate to heat up and heat water.Color hot water with yellow food coloring. Color cold water with blue food coloring. Fill cylinder about 2/3 full with cold water. Equations: Q = mc (delta T) Safety Issues: Hotplate and hot water |
|
4B20.10 Convection Tube Demonstrate heat transfer by by convection. Observe color spreading through the water as the warmer water stars moving. Setup Requirements: Assembled as needed. Fill with water. Heat one corner of lower part of tube using a candle. Add small amount of food coloring.
Equations: None
Safety Issues: Flame |
|
4B20.20 Two Chimney Convection Box Smoke is used to indicate convection in a two chimney box.
Setup Requirements: Minimal
Equations: None
Safety Issues: Flame |
|
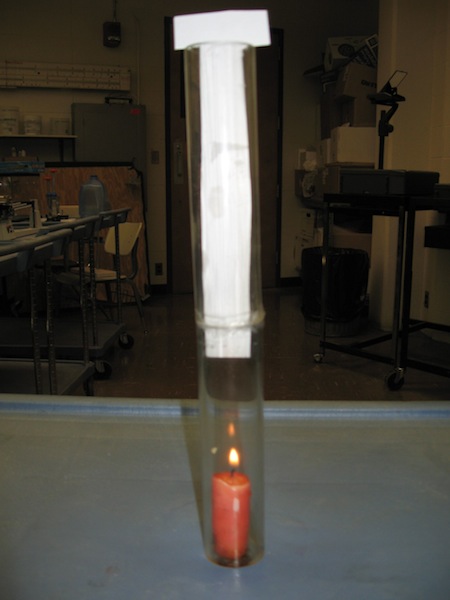
4B20.25 Candle in Tube (Symmetry Breaking and Convection). If a lit candle is covered with a long tube it will go out. All the oxygen near candle is used up. Rising hot gases prevent more air from coming in. Put a divider in middle of tube and try again. There is so much air flow it blows flame around. Some small random difference converts symmetric situation into one with hot gases going up one side and fresh air going down other side of tube. Setup Requirements: Minimal
Equations: None
Safety Issues: Flame,Smoke |
|
4B30.21 Heat Transfer by Conduction Using Metal Strips Demonstrate heat conduction. Put end of device in hot water. A strip along device changes color as temperature changes. Compare thermal conductivity of steel, brass, copper & aluminum. Setup Requirements: Some assemble required. Allow time for water to heat to boiling point. May want to turn on hot plate at start of class.
Equations: None
Safety Issues: Red hot hotplate |
|
4B30.22 Conduction Through Glass Demonstrate heat conduction. Hold lower bulb in your hand. Heat will cause pressure and volume of gas to increase. Fluid will move up into top of device. Setup Requirements: Minimal
Equations: None
Safety Issues: Fragile glass container. Do not squeeze tightly. Do not give to students. |
|
4B30.23 Conduction in Metal vs Foam Put ice cubes on two nearly identical blocks. One will melt much faster. One block is aluminum painted black to look like other material. Other material has low heat conductivity. Setup Requirements: Minimal. Ask ahead of time so ice will be available. Take a towel to wipe water off desk.
Equations: Talk about Q=mc(delta T), Q=mL, and conductivity. Heat conduction depends on type of material.
Safety Issues: None |
|
4B30.50 Frost on LN Cannon LN is placed in the bottom of the LN cannon. Frost will form starting at the bottom and extending up the tube. Use as demo of heat transfer by conduction.
Picture: Not Yet Available
Setup Requirements: Minimal. Ask at least 1 day ahead of time so LN will be available or bring your own LN.
Equations: None
Safety Issues: Liquid Nitrogen |
|
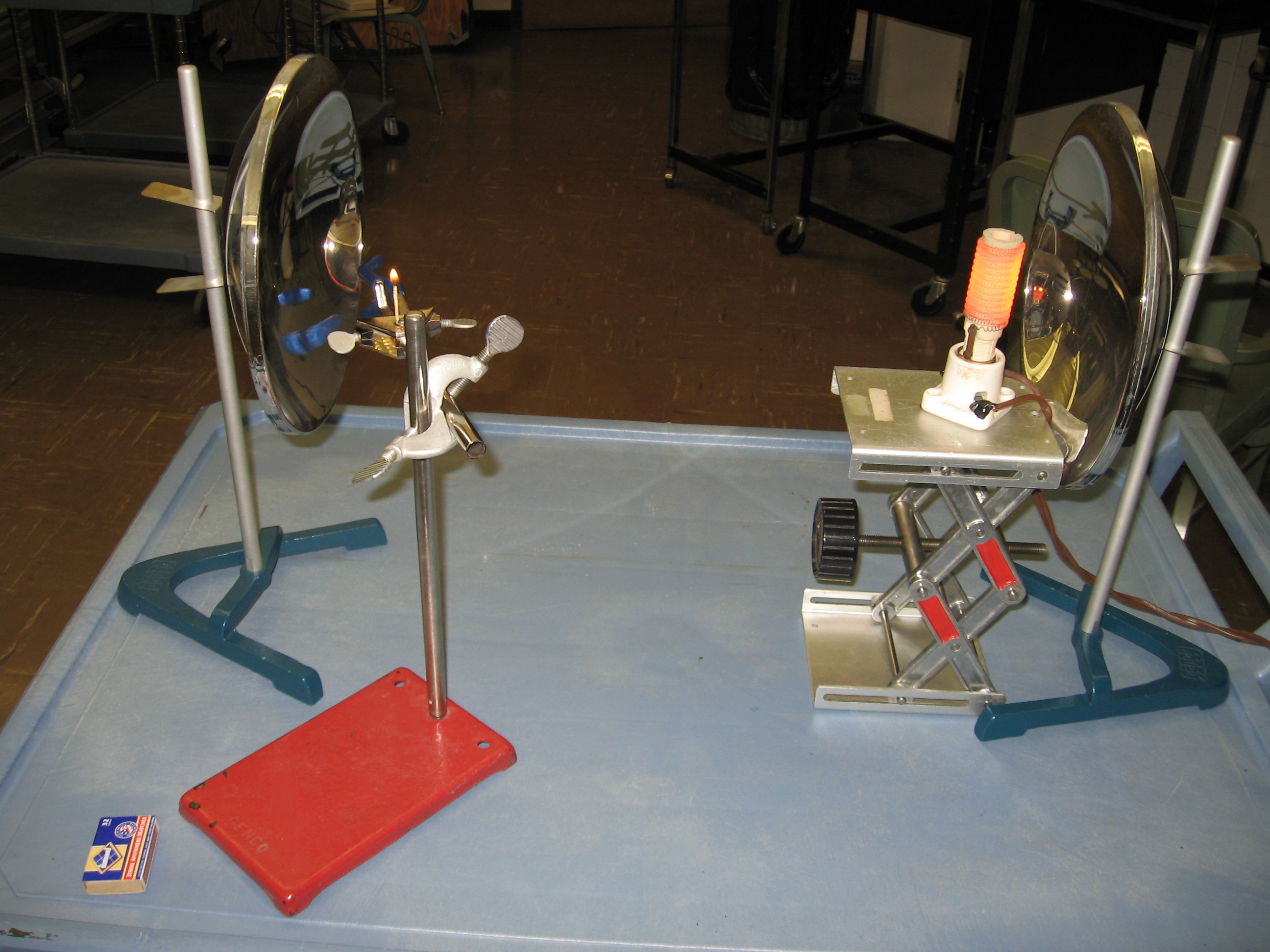
4B40.10 Light a Match the Hard Way NOT CURRENTLY AVAILABLE Use metal mirrors. Place lamp about 9-10 cm from concave mirror. Use jack stand to center coil. Place second mirror about 1 meter from first mirror. Use rods and clamps to place match about 9 -10 cm from second mirror. Adjust mirrors and match so light reflected by second mirror shines on the match head. The match will start smoking and then burst into flame. Setup Requirements: Assembled as needed on cart. Ask at least a day ahead. You will need to practice placing match at right place.Heater element shown in photo has been replaced by bright lamp. Use strike anywhere type matches.
Equations: None Safety Issues: Hot lamp. Hot enough to burn you at focus where match is located. Gloves available. Flame and smoke. |
|
4B40.15 Heat Transfer by Radiation Demonstrate heat transfer by radiation by focusing bright light on bottom of device. The heat will cause the air pressure and volume to increase and force a colored liquid up a tube into top of device.
Setup Requirements: Assembled as needed. Ask ahead of time
Equations: None
Safety Issues: hot light bulb, fragile glass device |
|
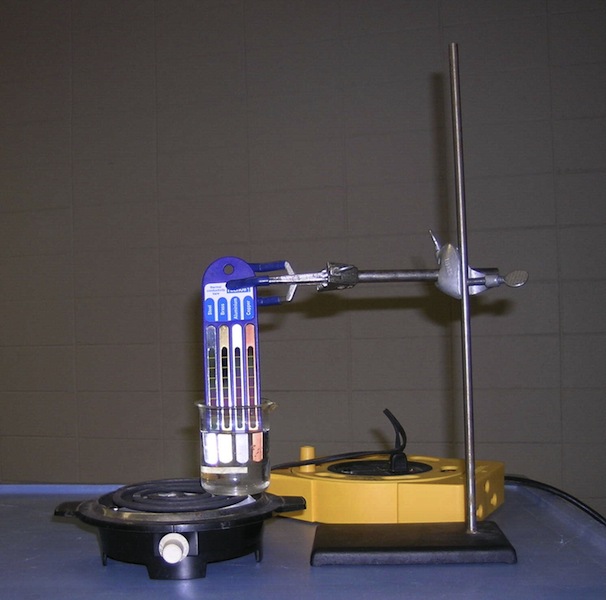
4B40.40 Good Absorbers & Good Emitters Demonstrate that good absorbers are good emitters. Put hot water in a silver can and a black can. Record temperature. Keep checking and recording temperature. The black can should cool faster. Setup Requirements: Minimal. Fill cans in Demo Room.You will need time to heat up water and take measurements. Suggest doing something else while letting water cool.
Equations: Radiative cooling depends on surface and fourth power of temperature (Kelvin).
Safety Issues: red hot hotplate, hot water |
|
4B50.20 Heating Water in a Balloon Demonstrate high heat capacity of water. Burn one balloon filled with air. Heat a second balloon filled with water.
Setup Requirements: Minimal
Equations: None
Safety Issues: Flame. Keep water filled balloon away from electronic devices on front desk. |
|
4B60.10 Heating Lead Shot Measure temperature of lead shot. Put in tube and shake tube of lead shot back and forth about 50 times.Measure temperature after shaking. The effect is small (~1 deg C). Use digital thermometer. Setup Requirements: Minimal. Need digital thermometer. Tube has changed from that in picture.
Equations: None
Safety Issues: Wash hands if touch lead. |
|
4B60.15 Kinetic Energy to Heat Put a piece of paper over large metal ball and hit it with the other ball. Enough kinetic energy will be converted to heat to burn a hole in paper. In smaller classes do several times and pass paper around so students can smell that paper is burned not just torn. Setup Requirements: Minimal
Equations: Kinetic Energy converted to heat (Q = mc deltaT).
Safety Issues: Dropping heavy balls. |